
 |  |  | 12.3 Benthic mineralization |
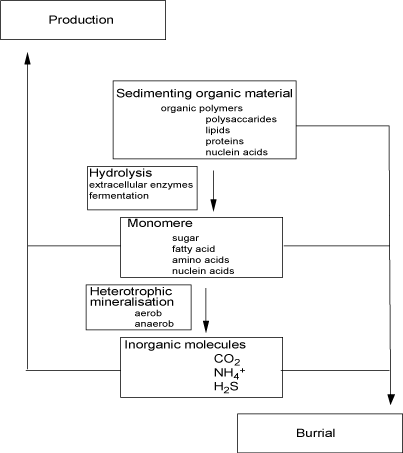
Alcohol fermentation:
C6H12O6 -> 2 CH3CH2OH + 2 CO2
Homolactic fermentation:
C6H12O6 -> 2 CH3COH2COO- + 2H+
As can been seen some Carbon atoms have been reduced while others have been oxidized during the process but overall there is redox-balance between the two sides of the equation and no net oxidation has occurred. Only a minor fraction of the available energy is released by fermentation, but it forms small molecules like acetate and propionate that can be used by the majority of the microbes for further degradation. Inorganic fermentation processes also exist and is in some instances of importance especially during methanogenesis and sulfur cycling but will not be dealt with in any detail.
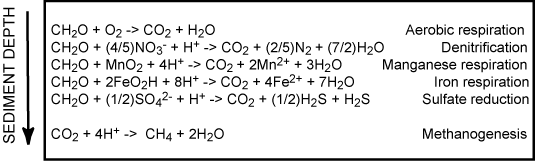
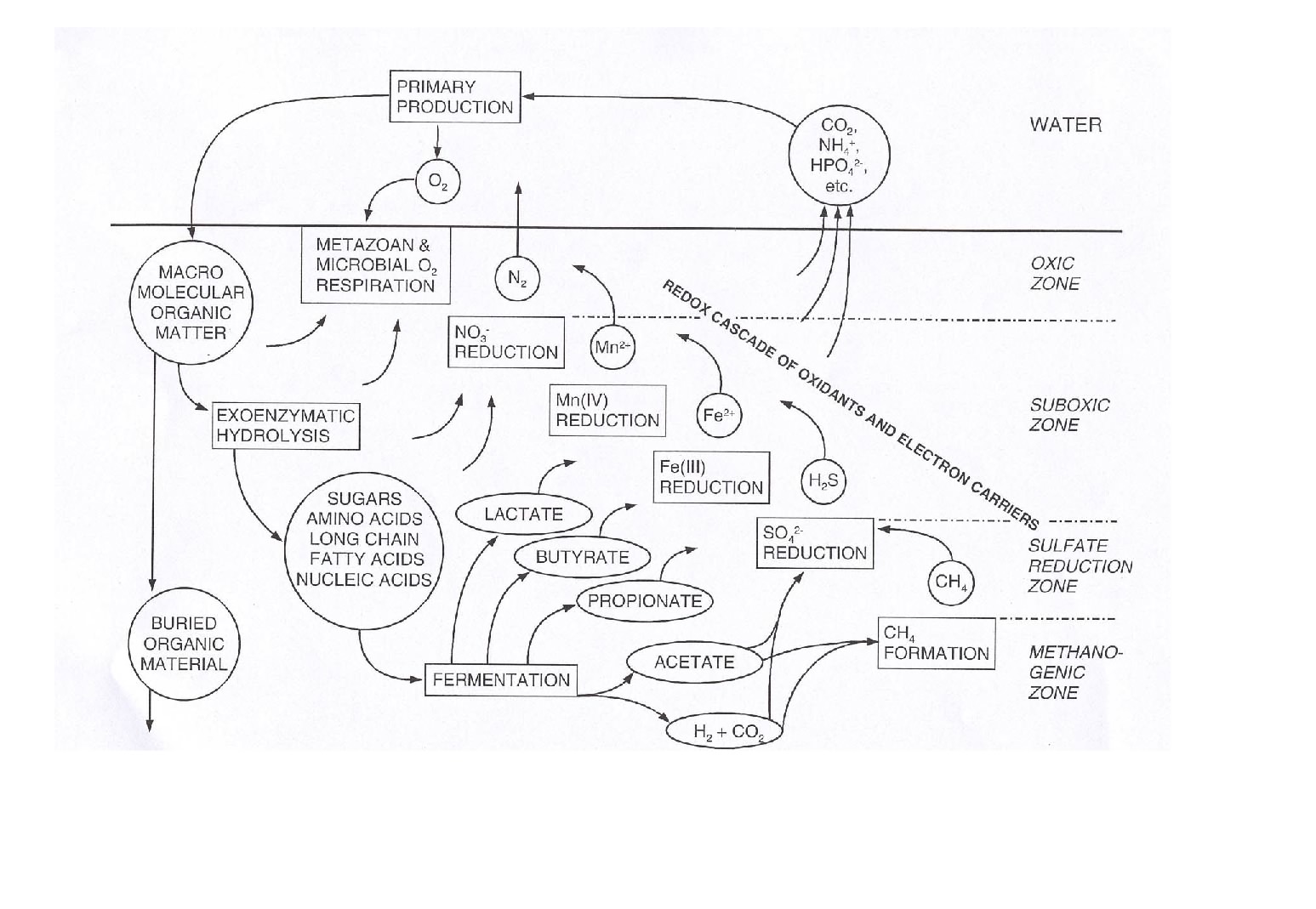
The vertical position of the anaerobic degradation pathways are mainly determined by the chemical energy liberated by the redox-process. Therefore denitrification is followed by metal respiration, sulfate reduction and methanogenesis. This laminated sequence is of course a simplification and often there exist overlaps between the different zone and microniches mainly induced by burrowing infauna or uneven sedimentation in the form of aggregates or fecal pellets exists, but essentially the various degradation pathways are sequential structured as outlined below.
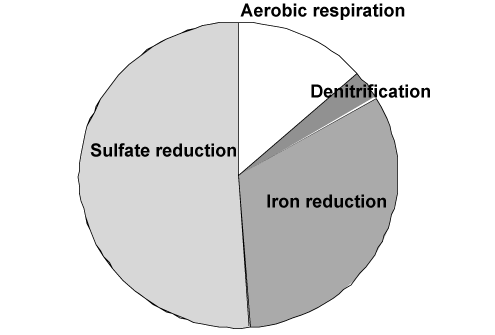
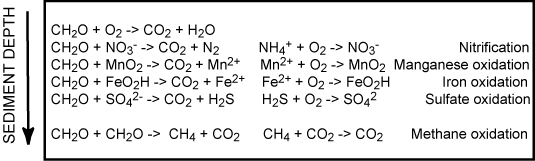
The importance of the various pathways depends on the input of organic material to the seafloor and the availability of the involved oxidants. In most coastal environments aerobic respiration only plays a relatively minor role but moving towards the deep sea where the input of organic material gradually decreases the importance of O2 as an oxidant becomes increasingly important. This is also reflected in the O2 penetration depth which broadens from a few mm in coastal areas to several cm or dm in the open oceans. Due to the low concentration of NO3- in most aquatic environments, denitrification is almost negligible for the degradation of organic material (even though it is a very important process for the aquatic nitrogen cycle). Due to an efficient chemical reduction of maganeseoxides (see below) and the generally low concentrations of manganese in sediments, manganese respiration also only plays a minor role for the benthic diagenesis - even though exceptions exist (see below). The importance of iron respiration for the degradation of organic material has been increasingly recognized during the last decades and it is a major pathway in many coastal environments. This is very explicitly in coastal areas receiving major inputs of terrestic ironoxides like fjords of tropical rainforest. For the vast majority of coastal environments sulfate reduction is the major pathway of benthic carbon degradation. This is due to the very high content of sulfate in sea water, which is in the order of 27 mM (at 34 ‰seawater) or around 100 times more than the O2 content of air saturated seawater (at 10°C). In marine environment methanogenesis is of less importance, but in limnic settings with their low concentrations of sulfate it can be a quantitatively important process. Strictly speaking most of the methane production in aquatic sediments proceeds by inorganic fermentation: 2CH2O -> CO2 + CH4, and is actually not a heterotrophic process.
The illustration above reflects the typical importance of heterotrophic processes in coastal sediments. However, it is important to notice that this is a theme with many variations and the relative importance of the various pathways many differ significantly between environments [460].
H2S + 2O2 -> SO42- + 2H+
HS- + O2 + H+ -> S0
S0 + H2O + 1 O2 -> SO42- + 2H+
They can store elemental sulfur (S0) intercellular for further redox processes when the environmental settings changes. By their chemosensory behavior these bacteria are capable of maintaining themselves within the O2 -- H2S interface typically characterized by very steep concentration gradients.
Combining the heterotrophic and chemolithotrophic processes introduced above gives a number of coupled redox processes as outlined below
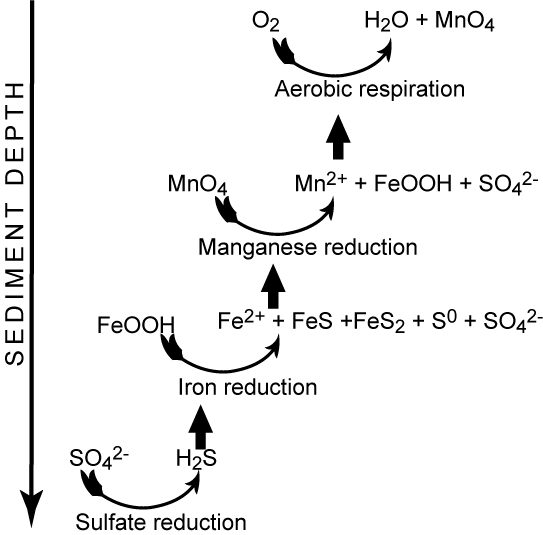
Since most of the reduced counterparts from the anaerobic degradation ultimately get reoxidized by O2 the total O2 uptake of the sea floor is the most widely used measure for the benthic diagenesis. This procedure assumes steady state conditions within the sediment. In reality the scheme outlined in Figure 5 is an oversimplification. Most of the reoxidation proceeds though a redox cascade rather than direct aerobic activity.
Most coastal sediments contain significant amounts of ironoxides and in those instances H2S produced by sulfate reduction reacts with these oxides producing a number of reduced ironsulfides. Pyrite (FeS2) is very stable under anoxic conditions and represents a burial unless the material is brought up to the oxic environments by for instance bioturbation. The reduced ferrous iron (Fe2+) and ironsulfides can react with manganeseoxides forming sulfate and ironoxides. In fact most manganges oxidation in marine sediments occur through this reaction and therefore manganese respiration in most sediments only plays a minor role.
Since ironoxides react with reduced sulfur they play a significant role in the benthic diagenesis and are of prime importance for the chemical (and biological) environment of the seafloor. Sulfide is toxic to higher fauna (and flora), but as long as the sediments contains ironoxides the sulfides do not escape to the overlying water but is "inactivated" by the so called ironoxide-layer. The amount of sulfide that sediments can inactivate is called the sulfide-buffer-capacity (SBC). The SBC decreases during spring and summer where sulfide production (sulfate reduction) gets stimulated by elevated temperature and increased loading of organic material. If the SBC is overloaded the sulfide front will start moving up to the sediment-water interface where sulfide oxidizing bacteria represent the last barrier towards sulfide release to the overlying water. Ironoxides have a large capacity to bind phosphor, and as they gets depleted phosphor is released to the overlying water, which may further stimulate the pelagic production and the sedimentation of organic carbon. The layer of ironoxides thereby represents an important zone buffering sulfide and phosphor release to the overlying water. A buffering that diminishes and ultimately vanish as eutrophication increase.
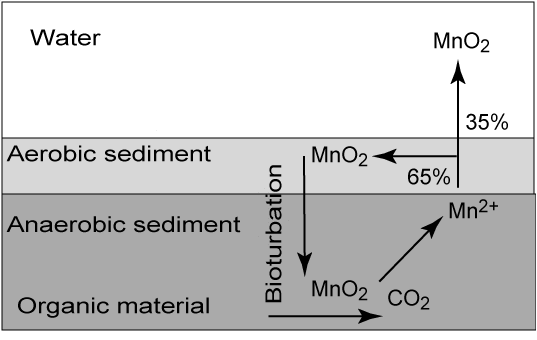
Metal oxides (FeO2H, MnO2) are solids and are not being transported by diffusion as the reduced counter parts (Fe2+, Mn2+). Therefore as the reduced solutes reach more oxidized conditions they precipitates and can only act in the heterotrophic carbon mineralization if they are transported to deeper layers either by bioturbation, resuspension or simply by burial. Fauna activity thereby plays an important role for efficient metal cycling in sediments. An example for manganese cycling in coastal sediment is displayed below
As can be seen some of the reduced manganese irons escapes to the overlying water, where it spontaneously reacts with O2 forming precipitates that can be transported with bottom currents to sites of deposition. Large amounts of manganese are deposited in central Skagerrak (700m), and the carbon mineralization is these sediments are completely dominated by manganese respiration due to the very high levels of available manganese oxides [69].
The figure below give an overall impression of the benthic carbon degradation of marine sediments combining most of the processes mentioned above.
 |  |  | 12.3 Benthic mineralization |