 |  |  | 14.3 The case of the silicate pump in the Bay of Brest |
14.3 The case of the silicate pump in the Bay of Brest
Evidence is growing that the nutrient silicic acid (DSi) is playing a
major role in the functioning of coastal ecosystems in many regions of
the world [104]. The reason is linked to the importance of
diatoms in marine food webs [109], and to anthropogenic
influences on watersheds and rivers. When DSi is missing, diatoms
become replaced by other phytoplankton groups that do not have any
requirement for this nutrient, such as dinoflagellates [347].
A wide variety of coastal ecosystems has been documented, where
increasing frequency and magnitude of harmful algal blooms have been
associated to decreasing Si:N and Si:P ratios, with important
consequences for pelagic and benthic food webs [430].
Decreasing Si:P and Si:N ratios first find their origin in
eutrophication. Urbanization, agricultural and industrial activities
have led to large increases in the delivery of N and P along the
land-ocean continuum. On a global basis, the fluxes of these elements
to the oceans have increased by a factor two; at the same time, in
rivers unaffected by human activities, DSi fluxes have remained
constant, as the major source of DSi to rivers comes from natural
silicate rock weathering [311]. The second source of
decreasing Si:N and Si:P ratios is river manipulation,
especially the build up of dams [236]. In the
reservoirs behind the dams, growth and sedimentation of diatoms remove
biogenic
silica (BSiO2) from the water column, leading to decreased DSi
concentrations [104]. Whatever the type of perturbation,
decreasing Si:N and Si:P ratios in rivers imply potential DSi
limitation for diatoms [137], which becomes true limitation
when DSi concentrations decrease below the half saturation constants
in the receiving coastal water bodies (e.g. [337]).
The Bay of Brest is an ecosystem where Si:N
ratios in riverine inputs have decreased by a factor of 3 in the past
30 years, mostly due to excessive N inputs from agricultural practices
[283]. Indirect evidence of DSi limitation has been provided,
on the basis of declines in diatom populations coinciding with DSi
concentrations becoming lower than 1 µM by early spring
[385]. DSi limitation has then been directly demonstrated
from kinetic uptake experiments using the 32Si radioactive
isotope [126]; see also Figure 2.
Despite DSi limitation during spring, diatoms typically continue to
dominate the phytoplankton during the entire productive period
[127]. Several factors have been hypothesized to account for
this (apparent lack of) response of the Bay to excessive N inputs.
They include the export out of the bay of most of this N during winter
[283], the well-mixed nature of the water column which does
not favor the development of flagellates [386], and the
intensity of Si recycling both in the water column and at the
sediment-water interface [385][127].
Although Si recycling has recently been shown to be accelerated under
high bacterial activity [44], it remains slower than the
recycling of N and P, which are biologically mediated [347].
In the open ocean, this differential recycling
rate is at the basis of the so-called silicate pump [140],
which removes DSi from surface waters for a long time period. In
coastal waters, especially in the semi-enclosed Bay of Brest, the
effects of the silicate pump may well be reversed, because of the
tight temporal and spatial coupling between sediment and surface
waters: following the first diatom blooms and the sedimentation of
diatom cells, Si can be retained within the Bay, at the sediment-water
interface, instead of being exported to the adjacent coastal ocean;
it then becomes directly available for
regenerated diatom production, because of the shallow depths of the
well-mixed waters [127].
Synthesizing 20 years of studies of the Bay of Brest ecosystem, both
from a pelagic and benthic point of view, Chauvaud et al.
[84] suggested that the functioning of this coastal
silicate pump is under the control of benthic suspension-feeders.
Suspension feeders dominate the benthic megafauna in the Bay of Brest
[467]. Introduced in 1950, the gastropod Crepidula
fornicata is now the main benthic suspension feeder in the Bay
[82]. Chauvaud et al. [84] have
suggested that increased suspension-feeding activity during early
spring (filtration and subsequent production of enormous quantities of
biodeposits) could lead to an increase in the temporary retention of
BSiO2 in the sediments of the bay, thereby limiting the export
of Si out of the bay. Subsequent BSiO2 dissolution during late
spring and summer, enhanced by increasing temperature and bacterial
activity, would provide the necessary DSi required by diatoms to
maintain their dominance throughout the productive period. It is
essential to note that the enormous amount of biodeposits produced by
C. fornicata has no equivalent in the ecosystem.
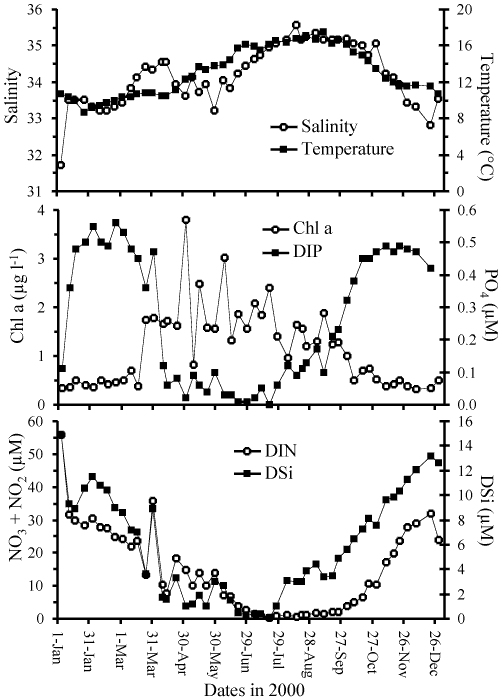
Figure: Physical, chemical and biological parameters measured
at the SOMLIT site in the Bay of Brest during the year 2000.
(a) temperature and salinity. (b) phosphate and chlorophyll
a. (c) silicic acid and nitrate. From Ragueneau
et al.
[382].
During the year 2000, the hypothesis of a "biologically active
silicate pump" was tested. Figure 3
shows the physical, chemical and biomass parameters recorded weekly at
the monitoring SOMLIT station located near the bay entrance. These
parameters characterize the productive season which begins in late
March in the bay with the increase in chlorophyll a corresponding to
the decrease in nutrient concentrations. A succession of
phytoplankton blooms occurred throughout the spring and summer. By
late July, DSi and DIP start to accumulate again in the water column,
followed by DIN two months later, when the productive period ends.
Direct evidence of DSi limitation during spring has been obtained
through two kinetic experiments performed when the diatoms
Rhizosolenia sp. and Chaetoceros sp. where
dominating the phytoplankton population (Figure
4). Having similar Km values close
to 1.3 µM, these diatoms were both limited to only 20% of
their maximal uptake velocity by late spring/early summer. Note that
Chaetoceros sp. exhibited a Vmax/Km ratio ten
times higher than that of Rhizosolenia sp., suggesting a
higher affinity and thus, a higher ability to take up DSi at low
concentrations. Thus, diatoms were clearly limited by ambient DSi
concentrations and were dependent upon Si recycling during early
summer. By late summer, DSi was accumulating again in the water
column (Figure 3), suggesting that DSi
inputs exceeded the diatom demand by that time.
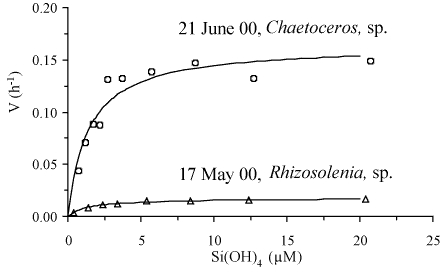
Figure: Two kinetic experiments performed on 17 May 2000 when
the diatom
Rhizosolenia was dominating and on 21 June
2000 when the diatom
Chaetoceros was dominating.
Experiments have been performed using the
32Si
radioactive isotope
[469]. A 3L water sample has been
distributed into eight 250 ml polycarbonate incubation
bottles. These bottles have been enriched with silicic acid
up to 20 µM, spiked with
32Si and incubated for
24 hours at light saturation. Following liquid scintillation
counting
[281], the specific uptake rate (V) is
plotted against the Si(OH)
4 concentration of the flasks
at the beginning of the incubation. These Michaelis-Menten
type of curves have been fitted using the non-linear
regression method of Wilkinson
[497], allowing
the determination of the maximal uptake velocity (V
max) and
the half saturation constant (K
m). From Ragueneau
et al.
[382].
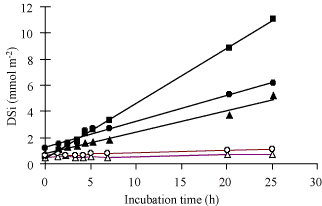
Figure: Sediment core incubation experiments conducted during
late summer in the Bay of Brest. Sediment cores were
collected at Rozegat using diving, at two sites located within
300 m but exhibiting contrasted densities of the suspension
feeder
Crepidula fornicata. Filled symbols: site
Rozegat with high densities (1243 ind. m
-2) of
C. fornicata,
[467]. Open symbols: site
Rozegat without any
C. fornicata. Note the factor of
20 between the mean flux measured at the site with
C.
fornicata (triplicates, mean: 6.3 mmol Si m
-2
d
-1) and the mean flux measured at the site without
C. fornicata (duplicates, mean: 0.3 mmol Si m
-2
d
-1). From Ragueneau
et al.
[382].
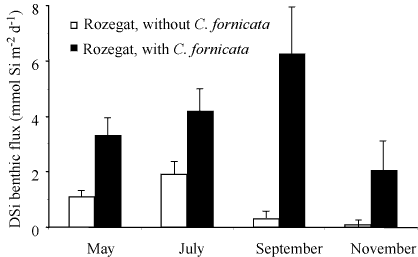 ragFig6
ragFig6
Figure 6. Synthesis of DSi benthic fluxes measured at the two
constrasting sites during the productive period in the Bay of
Brest. Black bars: site Rozegat with
C. fornicata;
white bars: site Rozegat without
C. fornicata. These
fluxes represent the mean values of the fluxes measured in
triplicates (see Figure
3.
 ragFig7
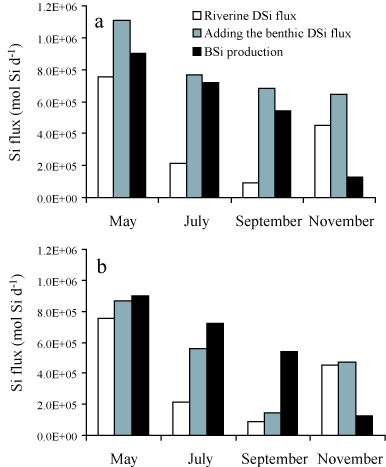
ragFig7
Figure 7: Seasonal budgets of DSi fluxes in the Bay of Brest.
All data in mol Si d
-1. White bars: river fluxes. Grey
bars: sum of river and benthic fluxes. Black bars: estimates
of silica production. See text for explanations on budget
calculations. (a) Benthic DSi fluxes have been extrapolated
to the whole Bay by applying the fluxes measured at each site
(Figure
4) to half of the Bay, i.e. the
present extension of the invasive
C. fornicata. The
grey bars do not represent only the benthic fluxes shown on
Figure
4 and extrapolated to the whole Bay;
they represent the sum of the river and benthic DSi inputs,
which can be directly compared to the diatom demand (black
bars). (b) Same description, only
C. fornicata has
been artificially removed from the system by applying the
benthic flux measured at the site without
C.
fornicata to the whole Bay and not only to half of it. From
Ragueneau
et al.
[382].
Four budgets were made for the productive season (Figure
7a), neglecting DSi inputs from the
adjacent ocean, as they represent less than 5% of the diatom demand
during the productive period [385]. These budgets clearly
demonstrate the importance of suspension-feeder activity on the Si
cycle allowing for DSi to be available for diatom production during
late spring and summer. By early spring, river Si inputs can sustain
nearly 100% of the diatom demand; diatoms do not depend upon
recycling at the sediment-water interface, especially if we add the
winter stock of DSi that can account for one third of the initial
diatom demand [385]. By late spring, river inputs have
decreased by a factor of three and can sustain only 30% of the diatom
demand. The rest must be met by recycling at the sediment-water
interface. By mid-summer, river inputs are even smaller and DSi
benthic fluxes alone can sustain diatom demand. Because recycling
also occurs in the water column, DSi is probably available in excess
and starts to accumulate in the water column (Figure
3). Note that in September, DSi inputs
exceed the diatom demand by about 140×104 mol Si
d-1(Figure 7a). The Bay volume is
close to 2×109 m3 on average, which means that DSi
should accumulate at a rate of roughly 0.07 µmol L-1
d-1. From late July onwards, DSi increases linearly from 0 to
12 µM in 5 months (Figure 3). This
corresponds to a rate of 0.08 µmol L-1 d-1, which is
very consistent with the above budget calculation. During fall,
diatom demand decreases sharply whereas benthic fluxes are still high
and river inputs have increased again due to rainfall. DSi continues
to accumulate in the water column at the mean rate calculated above
and will soon reach its winter maximum concentrations. The budgets
presented demonstrate unambiguously the importance of recycling at the
sediment-water interface in sustaining diatom demand throughout the
productive period.
 |  |  | 14.3 The case of the silicate pump in the Bay of Brest |
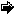





 ragFig6
ragFig6
 ragFig7
ragFig7