
 |  |  | 2.4 Transformation |
All chemical components which constitute nutrients in the ecosystem are reactive in one way or the other in the atmosphere. The chemistry is taking place on very different time scales and is highly complex. It is crucial to include the most imporant chemical transformations in the pathway description if the contributions of nutrients are to be described as accurately as possible.
In the chemistry of air pollution one distinguishes between primary and secondary air pollutants. Roughly it can be said that primary pollutants are directly emitted from the sources and in the air they transform by chemical reactions into secondary pollutants which are subsequently deposited. In the case of nitrogen, the primary pollutants are NH3, NO and NO2 and the secondary pollutants are NO2 and all the other nitrogen containing components that are produced from the primary pollutants in the air. Most important reaction products for the nutrient deposition are components containing nitrate (NO3-) or ammonium (NH4+) in particulate phase.
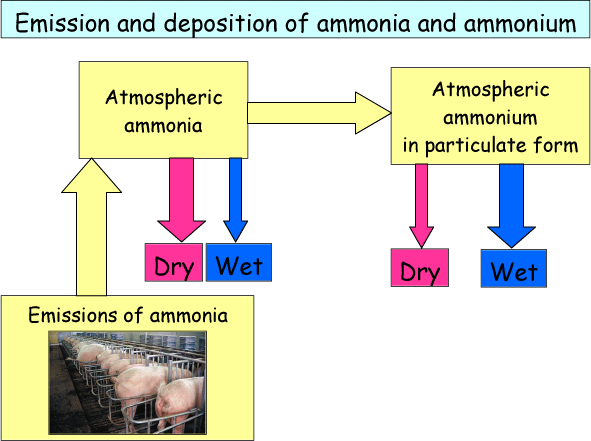
In Figure 5 the pathways for emission and deposition of NH3 and NH4+ are shown. NH3 is emitted in agricultural processes and is then present in the atmosphere in gaseous form. NH3 quickly dry deposits close to the source, however some of it is also transformed to NH4+ in particulate form. Particulates have a long lifetime in the atmosphere (≈one week) and can therefore be transported across long distances. The most important removal pathway for NH4+ is wet deposition.
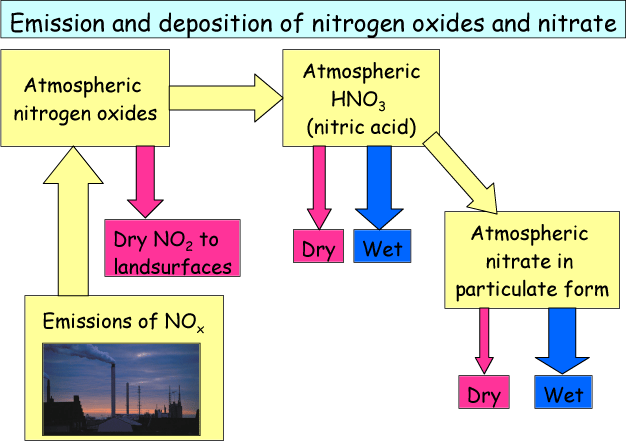
In Figure 6 the corresponding pathways for emission and deposition of NOx and NO3- are shown. NOx is emitted in combustion processes and is then present in the atmosphere in the form of gaseous NO and NO2. NO is slowly transforming into NO2 by reacting with oxygen in the atmosphere (this is why NO2 is both a primary and a secondary component), and some of the NO2 dry deposits close to the source. Some of the NO2 and NO transforms into nitric acid (HNO3) which subsequently transforms into NO3- in particulate form. As for ammonium the lifetime of particulate nitrate can be quite long and the most important removal pathway is also wet deposition.
There are many chemical components in the atmosphere, the total number has been estimated to be around 3000. The majority is reactive and the approximate number of different reactions taking place between these approximately 3000 species is ca 20,000. Reaction rates typically depend on temperature, pressure, sun angle and concentration of the chemical components. Due to this complexity of the chemical atmosphere, it is not straightforward to predict what will happen in terms of chemical reactions with the emitted nitrogen before it deposits again somewhere else.
 |  |  | 2.4 Transformation |