

 |  |  | 14.4 Ecological and biogeochemical implications |
Until quite recently, the Bay of Brest did not experience any sign of "true eutrophication", and more subtle shifts towards non-diatom species have been sparse. Our results suggest that it is the proliferation of the invasive C. fornicata which may well have masked for years the potentially negative effects of elevated N and P inputs, through its role on the coastal silicate pump mechanism.
It has been suggested that C. fornicata be removed from the ecosystem, since this invasive species has drastically reduced the native P. maximus fishery in the Bay of Brest. A theoretical removal of C. fornicata was made in 2000 by applying the DSi benthic fluxes measured at the site without slipper limpets (Figure 6) to the whole Bay of Brest (Figure 7a). The budget suggests that during summer DSi inputs from rivers and from the sediment-water interface would not be sufficient to sustain diatom demand. Diatoms would then probably be replaced by other algae not requiring DSi leading to the potential of harmful algal bloom with severe consequences for pelagic and benthic food webs. The massive bloom of the toxic dinoflagellate Gymnodinium nagasakiense, encountered during the summer of 1995 in the Bay of Brest, illustrates this theoretical removal scenario. C. fornicata was present but inactive. Indeed, feeding activity has been deeply affected by the mass sedimentation of the diatom bloom that occurred by late spring, through either gill clogging or oxygen depletion [291][83]. As a consequence, biodeposits were produced in much lower quantities and Si was exported out of the Bay in the form of BSiO2, instead of being stored at the sediment-water interface as biodeposits. Less DSi was then available for diatoms during late spring and summer, especially as river inputs were particularly low, and G. nagasakiense dominated the phytoplankton throughout the summer [84].
The 1995 Gymnodinium event, explained by a complete stop of the silicate pump mechanism, had dramatic effects on benthic food webs. In particular, it has led to a major interruption in the growth of P. maximus [84][291], and to differential larval and/or post-larval mortality, depending on the species sensitivity to toxic substances [85]. Interestingly, most indigenous species are more sensitive to Gymnodinium toxicity than the introduced mollusk [82], suggesting that nutrient enrichment would indirectly favor the successful colonization by non-indigenous species.
It is important to note that the silicate pump mechanism described herein has important implications for the ecosystem functioning, primarily because of DSi limitation induced by excessive inputs of N compounds from the watersheds. An interesting project is starting in the Bay of Brest, associating environmental and social sciences. Truely, C. fornicata endangers benthic biodiversity [84] and the development of P. maximus [467], which has important social and economical implications. At the same time, the proliferation of C. fornicata had beneficial effects for phytoplankton dynamics in the bay, reducing the effects, for phytoplankton dynamics in the bay, of the Si:N distrophy observed at the river output. Thus, paysants working on the watersheds may well have a different perception of the invasion than the fishermen working in the Bay. Therefore, it will be very important, in the coming years, to further investigate the mechanism of the silicate pump in the Bay (how long will it be efficient?) and launch a large discussion among the various actors of the Bay, to ensure its sustainable development for all. The case study presented herein thus provides a good example of an integrated approach, from the watershed to the coastal zone and from environmental to social sciences, to study the response of an ecosystem to two of the seven stressors described by Cloern [96], namely nutrient enrichement and proliferation of invasive species.
The mechanism described herein is also affecting primary production seasonality, with important feedbacks for benthic food webs. The Bay of Brest is showing a long-term change in the seasonal timing of phytoplankton biomass development [84]. The spring blooms have become smaller in amplitude but the summer biomass has become higher. These subtle seasonal-scale changes might lead to positive or negative feedbacks in the benthic system and in other components of coastal ecosystems [185]. How these responses might be used as early-warning indicators of systemic responses to nutrient enrichment [96] warrant further investigations. Mass population of invasive mollusks are developing throughout the world as a result of human introduction. Biotic invasions are an important component of human-driven aquatic alteration as major agents of global change [298][202]. Invasive species often outcompete native fauna for food and space, in the process altering the interactions between multiple components in the affected ecosystem [82]. The most famous example is the invasion of San Francisco Bay by the asian clam, Potamocorbula amurensis in 1986 [75]. Within two years the clam had proliferated to more than 10×103 individuals per meter squared, and accounted for up to 95% of the benthic biomass in colonized areas [339]. In the northern basin of the bay, this dense population directly controls the phytoplankton biomass [93][94][293] and affects the zooplanckton community by predation, or food competition [265]. The appearance and proliferation of this species has altered the food web of the entire ecosystem [339][465]. The Bay of Brest and San Francisco Bay examples show how spreading of exotic species in the benthic ecosystem and eutrophication can be interconnected and how these species can alter the ecosystem functioning when the population is high enough to control primary production in the overlying water column [93][84], and alter the cycling of nutrients such as carbon [117][135], oxygen [143], nitrogen [114], phosphorus [25] or silica [84]. Benthic community compositions have been profoundly modified by the proliferating invading species, threatening the benthic biodiversity at the ecosystem scale [84]. Stachowicz et al. [441] demonstrated experimentally that increased species richness significantly decreases invasion success, thus the impact of mild eutrophication on biodiversity should be included in further studies.
Short-term, ecological, consequences of enhanced Si retention at the sediment-water interface, due to the proliferation of invasive species of suspension feeders, have been explored in Section 14.3 of this chapter. Long-term, biogeochemical, consequences will now be explored, discussing the so-called silica depletion hypothesis.
Both causes of this silica depletion hypothesis, eutrophication and river manipulation, involve enhanced diatom production, although the trapping of phytoliths in dam reservoirs could also play a role. Here, it is suggested that increased biodeposition by suspension feeders, as evidenced in Section 14.3 of this chapter, could constitute a third means of depleting water column DSi concentrations.
Diatoms are taken up by benthic suspension feeders in the process of feeding with regeneration of silicic acid from the biodeposits in the sediments [24]. Only very few investigations of bivalves and bivalve beds as sources and sinks of silicon exist in the literature [26][116][135][378] where high rates of DSi release are observed from sediments probably occurring through the silicate pump mechanism described above for the Bay of Brest [84][382]. Benthic filter feeders produce such high quantities of biodeposits that the subsequent dissolution of BSi allows for high rates of benthic DSi fluxes (see Section 14.3). However, due to the importance of biodeposition, even if only a small fraction of the BSi embedded in bivalve feces and pseudo-feces gets preserved, the accumulation of BSi may represent an important flux, which needs to be quantified on annual and interannual time scales. In this perspective, a preliminary budget of annual Si fluxes has been built in the Bay of Brest (Figure 8). The establishment of such a budget [383] is briefly described below, with a special emphasis on the demonstration that biodeposits are enriched in silica and constitute an environment favouring subsequent preservation of biogenic silica.
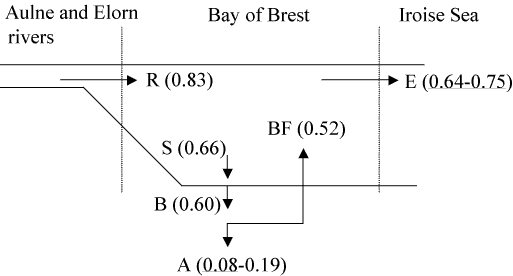
To provide an estimate of the silica enrichment factor in suspension feeders faeces, we have performed a simple experiment [321]. The proliferating gastropod C. fornicata, collected in the Bay of Brest, has been fed with a diatom Chaetoceros calcitrans after a 48 h period of starvation. A Si:C molar ratio of 0.049 ±0.002 was measured in the food. The Si:C measured in the feces after 24 hour was 0.118 ±0.002. Thus, in this experiment, the Si:C ratio has increased by a factor 2.4 between the phytoplankton and the faeces. The experiment described herein needs to be repeated with other bivalves and it remains to be established whether this result is due to the feeding activity of the bivalve itself, or to the associated bacterial activity. Whatever the mechanism, this result confirms that grazers actively participate in the decoupling between Si and C. Biodeposits are thus enriched in silica, suggesting that it would be important to determine how much of this BSi embedded in biodeposits, eventually gets preserved in the sediments. In the absence of direct measurements of annual Si accumulation data, we have performed two indirect, but independent, estimates of BSi preservation efficiency in the sediments of the Bay of Brest.
Figure 8 synthesizes the above calculations and provides a preliminary budget of annual Si fluxes in the Bay of Brest ecosystem. The important information to be derived from this budget is the role that biodeposition is playing in the system. It leads to the temporary retention of 72% of DSi river inputs. Of the BSi being bio-deposited, from 13 to 32% eventually accumulates on an annual basis. This preservation efficiency of the BSi embedded in biodeposits approaches that measured in the Southern Ocean (range from 8 to 40%, [384]), whereas that measured values in other deep-sea settings range from 5 to 15% [384]. It suggests that the sediment conditions induced by biodepositing organisms provide a means of efficiently preserving the biogenic opal.
The annual retention of Si in the Bay of Brest ranges between 10 and 23% of DSi river inputs. It is important to note that it would be much lower in the absence of the invasive species C. fornicata, which proliferation leads to both increased deposition rates and sedimentary conditions favorable to BSiO2 preservation. Sediment cores will soon be collected at sites exhibiting contrasted densities of C. fornicata, to test this hypothesis by reconstructing changes in the accumulation rates of sediment, organic C, BSiO2 and diatom assemblages.
As noted in Subsection 14.4.2, biological invasions will continue, as mollusk aquaculture production is growing rapidly (www.fao.org ) and expanding international commerce accelerates the translocation of fauna [74]. Thus, it will be important to test the role of increasing biodeposition in lowering the DSi water column concentrations, as it may already be very important in some ecosystems, and will most probably become even more important in the coming years.
 |  |  | 14.4 Ecological and biogeochemical implications |