|  |  | 14.2 Benthic-pelagic coupling and eutrophication |
14.2 Benthic-pelagic coupling and eutrophication
Oxygen deficiency in temperate coastal waters has led to an increased
awareness of vertical particle flux to bottom waters. In recent years,
particular attention has been paid to coupling and energy transfer between the benthos and plankton.
It has been postulated that
suspension-feeding communities can self-organize to enhance the
efficiency of food capture and thus establish boundary systems capable
of successfully exploiting the less structured planktonic system
[172]. Others studies demonstrated that in temperate coastal
waters the most prominent event in the annual flux of organic material
to the benthos is usually the spring diatom bloom [433][213][488][352].
In coastal (and also temperate and polar) seas this rapidly sinking
phytoplankton is often dominated by diatoms that reach the sea floor
relatively intact without being ingested by zooplankton (see
[433] for a review; [4]). Seasonally sedimented
phytoplankton blooms are a major source of nutrients that are
processed rapidly through the benthic system in open coastal areas
[172][183]. Information from field studies supports the
hypothesis that suspension feeders ingest a wide spectrum of particle
sizes [389][98][372]. Many suspension feeders are capable
of utilizing any type of food, and are limited only by morphological
constraints [394].
For the case of suspension-feeding bivalves, food quality and quantity
have been shown by Willdish and Kristmanson [496] and
Rosenberg and Loo [399] to be a limiting factor. For
example, infaunal bivalve growth was shown to be positively correlated
with both the chlorophyll input to the sediment and the diatom
availability in the near-bottom waters [466][297][496]. It
has been widely recognized that benthic suspension feeders, which are
among the main contributors to the biomass of benthic communities of
coastal and estuarine ecosystems world-wide, benefit directly from
pelagic primary production in the overlying water column
[183][88]. Thus, suspension
feeders are responsible for a considerable share of the energy flow
from the pelagic to the benthic system, in addition to secondary
production in benthic environments [370][172].
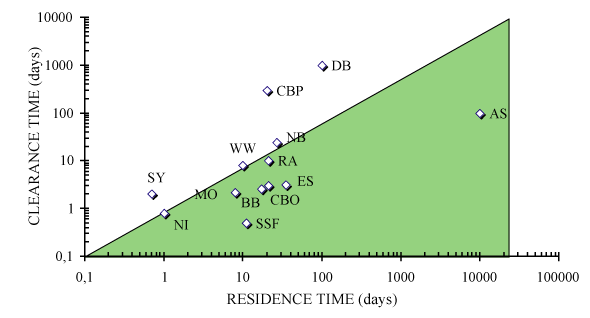
Figure: Graphical comparison of water mass residence time and
clearance time in suspension feeder-dominated ecosystems.
Ecosystems situated in the shaded area are potentially
regulated by suspension feeders where clearance time is
shorter than clearance time. AS: Askö Bay. SSF: South San
Francisco Bay. OS: Oosterschelde. CBO: Chesapeake Bay, past.
BB: Bay of Brest. MO: Marennes-Oléron Bay. RA: Ria de
Arosa. WW: Western Wadden Zee. NI: North Inlet. NB:
Naragansett Bay. SY: Sylt, Eastern Wadden Zee. DB: Delaware
Bay. CBP: Chesapeake Bay, Present. In Dame
[115]. From
Grall and Chauvaud
[185].
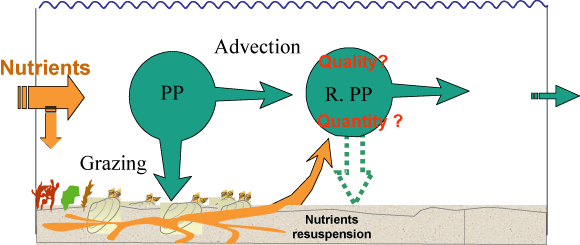
Figure: Schematic representation of the nutrient fluxes and
pelagic primary production dynamics in a suspension
feeder-dominated ecosystem. PP = Primary Production. RPP =
Regenerated Primary production. From Grall and Chauvaud
[185].
Oviatt et al. [358] note that as primary
production increases, respiration and thus recycling of nutrients in
the water column also increases. The pelagic production fuelled by
nutrients derived from the benthos could then result in higher pelagic
recycling rates. De Casabianca et al. [119]
argued that the concentration of dissolved inorganic nitrogen did not
vary much as a function of the presence or absence of a benthos, but
rather in recycling of nutrients either in bottom sediments or in the
water column. Thus, the effects of the benthos on pelagic production
are two fold: supplying nutrients directly, and indirectly increasing
regeneration rates in the overlying water column.
In the following section, we will explore the influence of benthic
suspension-feeders on phytoplankton dynamics, in an ecosystem (the Bay
of Brest, France) experiencing both excessive N inputs from the
watersheds (leading to silicic acid limitation) and the proliferation
of an invasive suspension-feeder, Crepidula fornicata (leading to
enhanced biodeposition). Short-term ecological and long-term
biogeochemical consequences of such an interaction will be explored in
Section 14.4 of this chapter.
 |  |  | 14.2 Benthic-pelagic coupling and eutrophication |